Welcome to eIFU
One Platform. Every Asset. Global Compliance.
A secure, scalable platform to manage and deliver structured content across regulated industries. From digital Instructions for Use (IFUs) and Product Leaflets (PILs) to multimedia artifacts like video guides, audio instructions, 3D models, safety data, and more — eIFU is your central hub for multilingual, traceable, compliance-ready assets.
Built for manufacturers, suppliers, and compliance teams, eIFU empowers you to publish, organize, translate, and distribute mission-critical content from a single source of truth.

Why Choose eIFU?
Flexible enough for niche teams. Scalable enough for multinational compliance.
Key Benefits:
- Supports life sciences, chemicals, industrials, food, agriculture, cosmetics, and more
- Compatible with IFUs, PILs, ePIs, MSDS, training videos, audio files, labels, and 3D drawings
- Structured XML, PDF, HTML, video (MP4/WebM), image (PNG/JPG/SVG), and 3D model (GLB/FBX) support
- Integrated multilingual publishing with translation handoff
- Public and private access controls
- Full version history and audit trails
- Regulatory-compliant workflows (e.g., EU MDR, REACH, GHS, FDA)
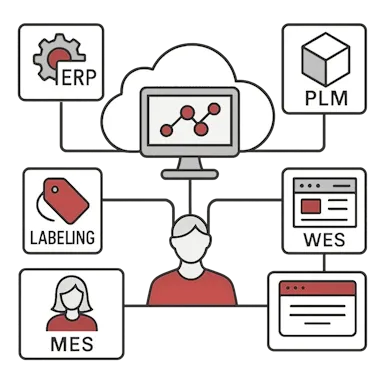
- Seamless integrations with labeling, ERP, PLM, and document control systems
Built for Real-World Use
Smart, scalable, and ready for any structured or rich content delivery need.
Asset & Metadata Hierarchies
Map files to products, markets, SKUs, categories, and territories.
Pre-Configured Templates
Out-of-the-box layouts for common document and media types — configurable for custom schemas.
Robust & Flexible Workflows
Route multilingual assets and files through approval and publishing pipelines.
Beyond PDFs. Built for Control.
eIFU is more than storage — it’s a global compliance command center for digital assets.

Seamless Connections. Zero Friction.
Connect eIFU to your ERP, MES, PLM, labeling, or media management systems. Eliminate manual updates by synchronizing product metadata and publishing events.
Cloud-Native. Enterprise-Ready.
Secure cloud deployment or on-premise options — built for enterprise-grade environments.
The eIFU Advantage
Fast Deployment, Faster Compliance
Fast Deployment, Faster Compliance
Accelerate launches and updates with reusable templates and automated routing.
Scale to Any Industry
Whether launching a new medical device, chemical, packaged food, or consumer product — eIFU adapts to your regulatory and informational needs.
Control Without Complexity
Manage a wide range of asset types with structured simplicity.